Overview
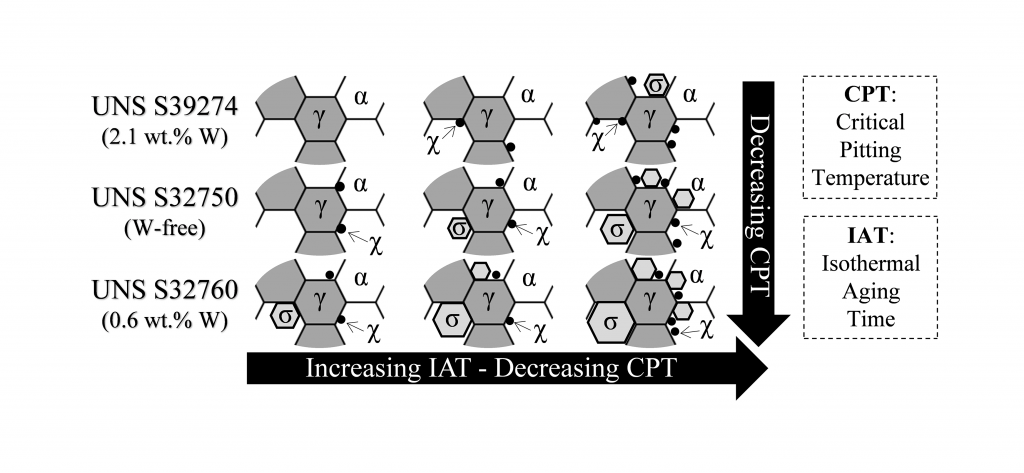
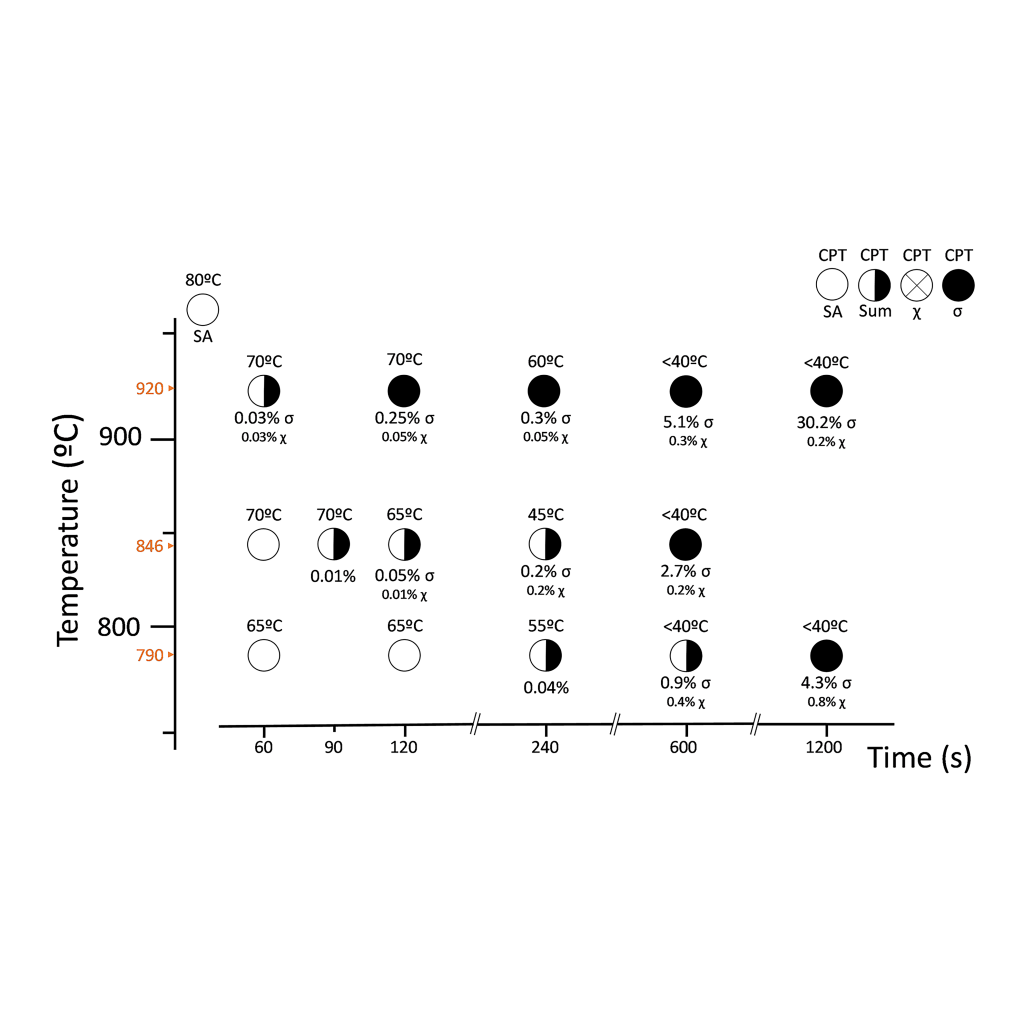
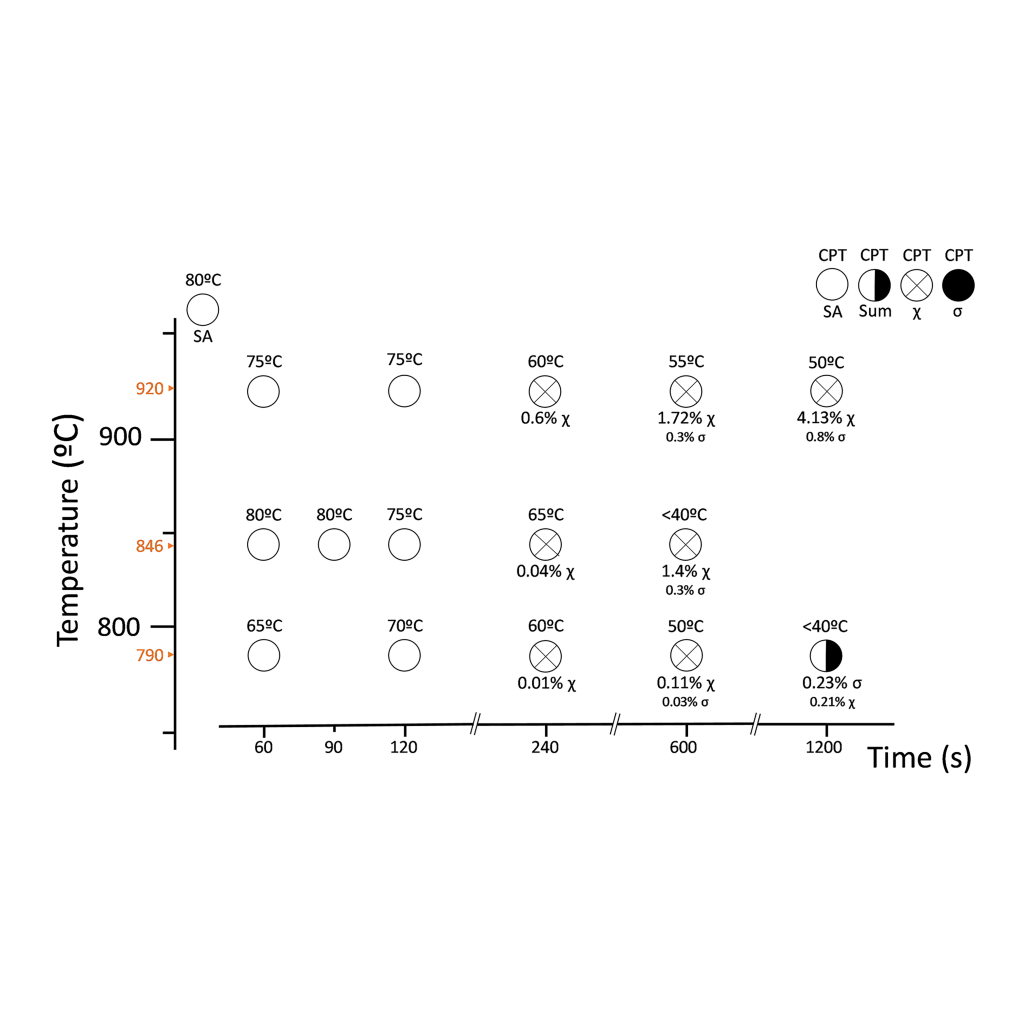
Super Duplex Stainless Steels (SDSS) have excellent corrosion resistance due to their high concentration of alloying elements like Cr, Mo, and N. There is still, however, disagreement on the role of tungsten in the corrosion resistance of stainless steels. In this regard, the influence of tungsten on tertiary phase precipitation kinetics remains a chief source of controversy. This investigation managed to reconcile the conflicting views by demonstrating that W plays different roles depending on the concentration range; it is added in solid solution. Thus, a 0.6 wt.% W addition accelerated sigma-phase (σ) precipitation kinetics, whereas 2.1 wt.% W additions retarded the precipitation kinetics of all tertiary phases—including σ-phase—favoring the formation of chi-phase (χ). Results showed that both χ- and σ-phase affected corrosion resistance, reducing the critical pitting temperature by 10-20°C at concentrations well below 1 vol.%.
Objectives
This investigation characterized the tertiary phase precipitation on three different SDSS differing in W content, namely, UNS S32750 (W-free used as reference), UNS S32760 (0.6 wt.% W), and UNS S39274 (2.1 wt.% W) that were isothermally aged at different temperature-time combinations. Additionally, the microstructure characterization was correlated to the corrosion resistance of the materials by means of the critical pitting temperature (CPT). The results were combined into visual Time-Temperature-Transformation-Corrosion (TTTC) maps.
Why is it important?
Tertiary phases can precipitate within the microstructure of stainless steels during processes such as welding and heat treatments. These phases can be deleterious, affecting both mechanical properties and corrosion resistance by introducing inhomogeneities in the microstructure. For instance, the main tertiary phases precipitating in duplex and super duplex stainless steels, σ- and χ-phase, are richer in Cr and Mo than the base alloy, creating depleted areas around them, which are prone to initiation of localized corrosion. The findings of this investigation showed that W can play a critical role in controlling the rate and type of tertiary phases in SDSS, which could be used to optimize, e.g., welding procedures.
Citation
C. Torres, M.S. Hazarabedian, Z. Quadir, R. Johnsen, M. Iannuzzi, Journal of The Electrochemical Society 167, 8 (2020): p. 081510. DOI: https://doi.org/10.1149/1945-7111/ab90af