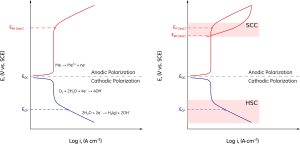
(left) schematic representation of anodic and cathodic polarization curves of a passive material or an alloy that develops a protective surface scale in an aerated corrosive electrolyte. The figure shows the electrochemical potential regions of the different anodic and cathodic reactions. (right) Schematic representation of the anodic and cathodic electrochemical potential regions where EAC is possible. SCC occurs at anodic potentials above ERP and slight above EP. HSC and SSC are exacerbated by cathodic potentials.